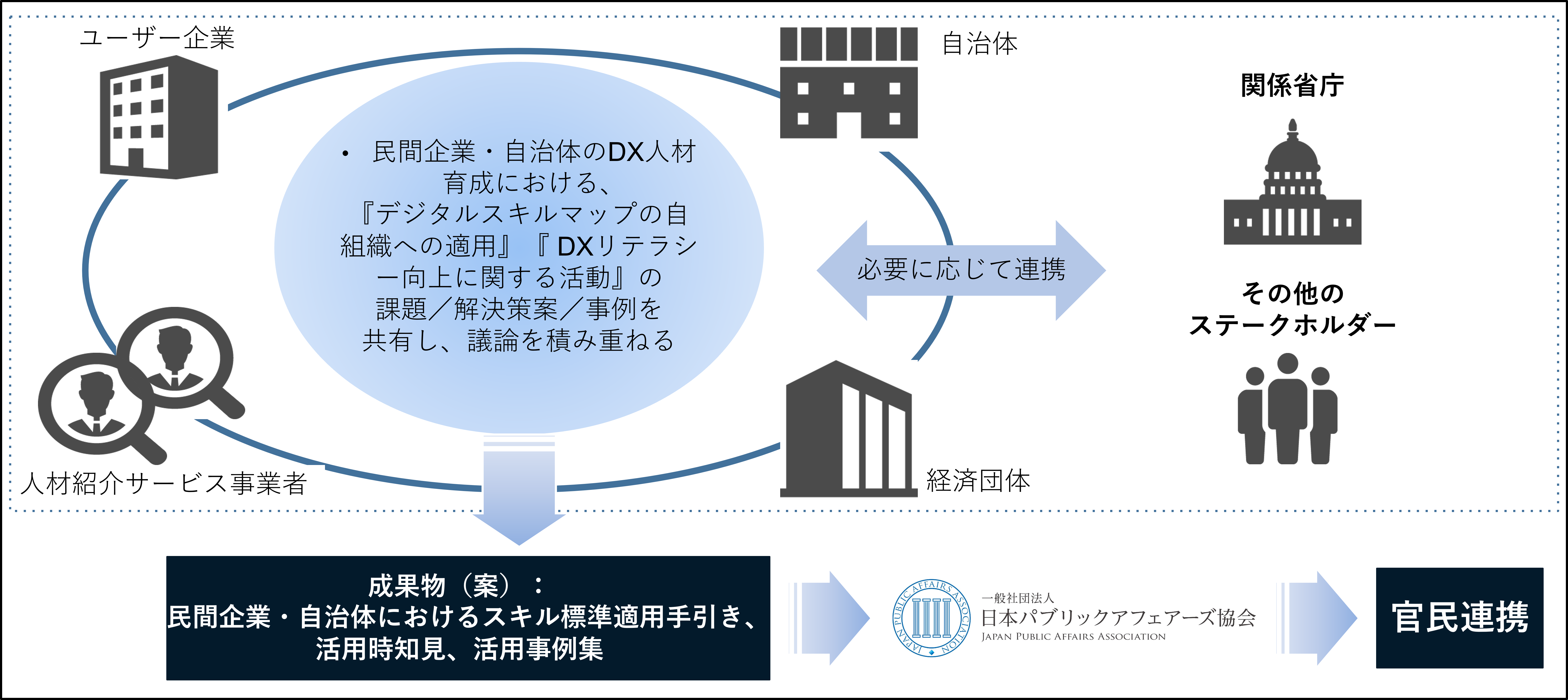
スイッチOTC医薬品推進に関する政策提言書(英語版)を発表

一般社団法人 日本パブリックアフェアーズ協会は、2023年11月7日に政策提言レポート『偽造医薬品横行の個人輸入問題と、スイッチOTC医薬品推進のための5つの提言』(英語版)を発表しました。
Summary
With the ongoing effects of the COVID-19 pandemic and access to healthcare facilities still facing restrictions, self-medication has gained attention. With this backdrop, the Japanese government has shown a proactive approach to the promotion of self-medication and has announced the “acceleration of Rx-to-OTC switch drugs” as a key policy. However, as of 2023, Japan’s universal health insurance coverage system and free access to hospitals has made it easy to visit a doctor even for minor illnesses, leading to a mere 6.9% of all drugs being over the counter (OTC), the lowest among G7 nations, with no significant signs of change. As a result, various issues have arisen, such as increased financial strain of the health insurance system, increased outpatient consultation times, and difficulty accessing drugs in a timely manner. In this policy proposal, we will cover the issue of private importation of drugs, which is often overlooked when discussing the promotion of self-medication and Rx-to-OTC switch. We hope to propose the direction to take the OTC drug approval system and self-medication promotion initiatives, from the perspective that the expansion of Rx-to-OTC switch and OTC drugs will help to accelerate the adoption of self-medication, as well as prevent damages caused by the private importation of counterfeit drugs.
The majority of privately imported drugs are not approved in Japan and some are counterfeit drugs. A common reason for private importation is the inability to buy the genuine drug easily at drug stores. We believe the cause for private importation is the limited availability of convenient methods to purchase genuine drugs, which in turn, can lead to the spread of issues caused by counterfeit drugs. In addition, the purchase of drugs online is becoming more commonplace, and is predicted to become more widely adopted, which is likely to lead to more health risks caused by privately imported counterfeit drugs. For this reason, it can be said that there is an urgent need to increase avenues to purchase genuine medicine through Rx-to-OTC switch, in order to protect citizens from off-label and counterfeit drugs.
We believe there are three main reasons for the unfavorable progress in Rx-to-OTC switch in Japan. First, is the lack of government goals and clear roadmap for Rx-to-OTC switch. Another factor is the ambiguity surrounding the specific topic of discussion that will be addressed at the Evaluation Council Regarding the Switch from Prescription Drugs to Drugs Requiring Guidance or Over-the-Counter Drugs (hereinafter, the Evaluation Council). Third, is the lack of a set deliberation deadline for the Evaluation Council. As a result, for example, discussions by the Evaluation Council regarding emergency contraceptives have lasted for a total of approximately six years.
In addition, the proposal will cover two possible issues that may arise due to the promotion of Rx-to-OTC switch. First, is the lack of a forum to discuss challenges and possible solutions regarding the promotion of OTC drugs, leading to the lack of a way to amass and share evidence regarding OTC drugs, and unclear direction when deciding how to best utilize OTC drugs in Japan. Another issue is the absence of a system that considers over-the-counter drugs being used by patients when prescribing medication, which increases the risk of prescribing drugs that may have adverse interactions.
With these challenges in mind, this policy proposal suggests the implementation of the following five policies.
Proposal
1.Establishment of a Rx-to-OTC Switch Roadmap Committee and the prompt development of KPIs and roadmap for Rx-to-OTC switch
2.Evaluation Council restructuring (implementation of a target timetable for considerations, clarification of discussion topics required to achieve KPIs, creation of a deadline from submission of written proposal to start of deliberation)
3.Creation of an OTC drug database
4.Creation of an OTC pharmaceutical record book in line with the self-medication tax system
5.Creation of a Japanese OTC Pharmaceutical Society

Five-Policy-Proposals-to-Combat-Proliferation-of-Counterfeit-Drugs-from-Private-Importation-and-Prom PDFファイル 2 MB ダウンロード

-scaled.jpg)